As industry prepares for the FDAs draft RWE guidance in 2021 Aetion conducted a systematic review of FDA approval documents in 2019 to understand what role RWE plays in informing regulatory decisions. ICER adopts Aetions Real-World Evidence Platform advancing standards for the use of transparent replicable real-world evidence in health technology assessment NEW YORK and BOSTON February 24 2020 Today the Institute for Clinical and Economic Review ICER announced it has selected Aetion as a preferred partner and platform to generate decision-grade real-world.
 Enabling Essential Evidence For Healthcare Sebastian Schneeweiss Md
Enabling Essential Evidence For Healthcare Sebastian Schneeweiss Md
The platform is able to isolate a subset of results that a specific intervention caused.

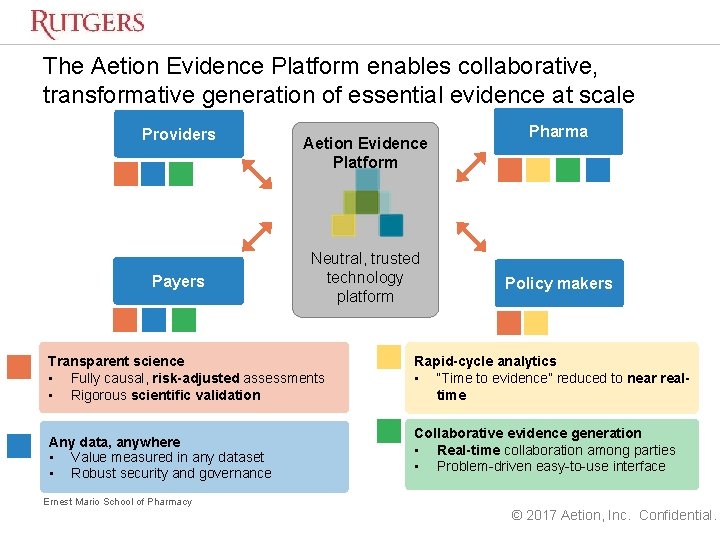
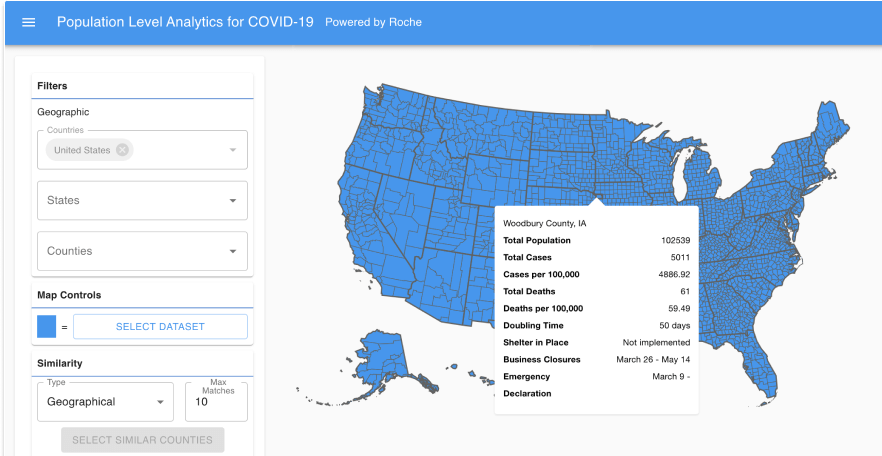
Aetion evidence platform. Aetion delivers real-world evidence RWE and outcomes-based analytics solutions to life sciences companies payers and at-risk providers. The Aetion Evidence Platform uses the everyday clinical and financial interactions of the health care system to unlock essential evidence about the effectiveness and value of treatments. Aetion a four-year-old health care startup that aims to bridge the gap between providers payers and biopharmaceutical companies with a novel analytics solution today announced that it has.
Das Unternehmen sagt es hofft zu helfen die FDA-Forschung die Krankheit den. Having this powerful ability companies using the Evidence Platform are able to make critical decisions that will save them time and money. Aetion erklärte in einer Presseerklärung dass die Partnerschaft sich auf der Unternehmens-Beweis-Plattform die gebaut um zu ermöglichen mehr Transparenz in der Berichterstattung und einfacher Austausch von Analyse und Wiedergabe von real-world evidence Ergebnisse.
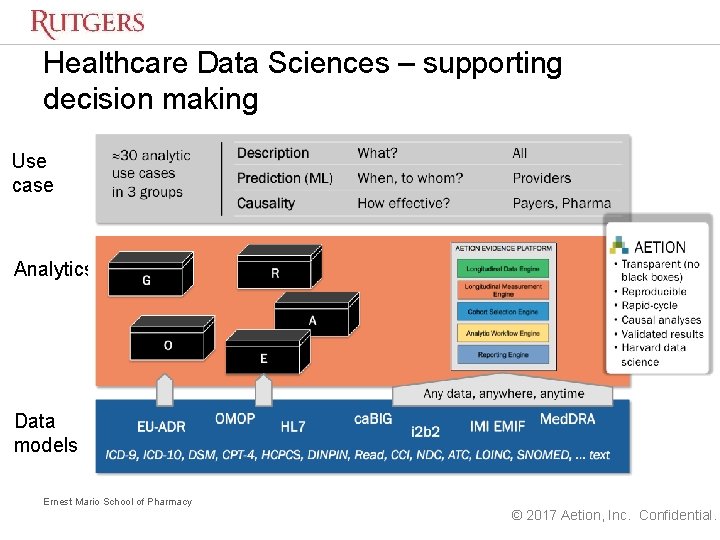
We achieve this with our Aetion Evidence Platform a software platform used to evaluate the safety effectiveness and value of medications delivering better outcomes to patients medical professionals and clients. What sets the Aetion Evidence Platform apart is the deep scientific expertise that has informed the technology along every step of the way. Food and Drug Administration now uses the Aetion Evidence Platform AEP as it develops more modern drug approval and safety.
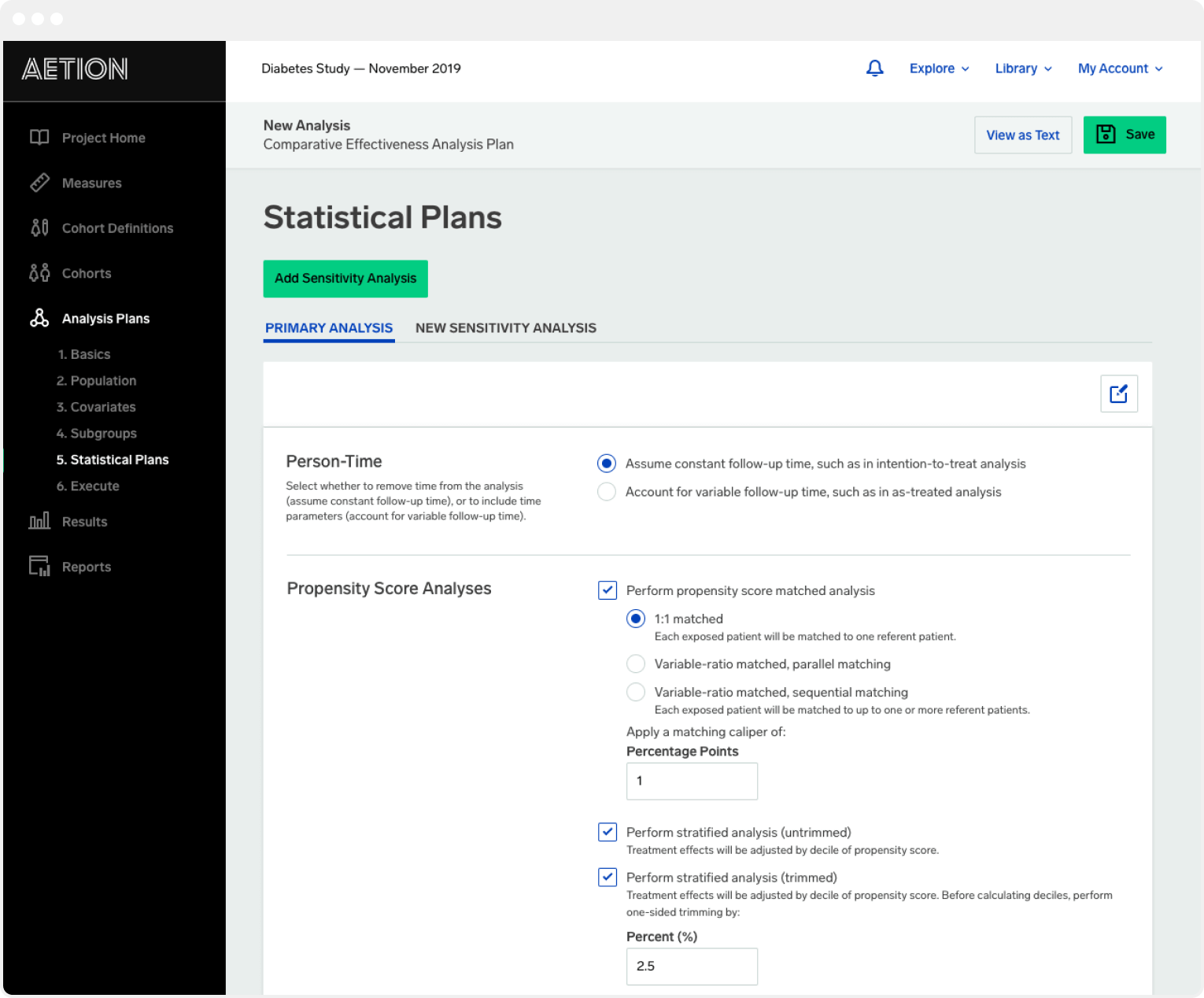
Just a few years after its launch the Aetion Evidence Platform is used by the majority of the top global biopharma firms leading payers and regulatory and HTA agencies to inform decisions on the. According to the company its goal is to help guide product development commercialization and payment innovation WHAT ITS FOR. It requires input from experts across data strategy data science biostatistics and advanced pharmacoepidemiology groups and a platform or program to actually execute the ECA.
Its validated rapid-cycle analytics allows users to significantly reduce time to evidence and improve patient outcomes. We look forward to continuing to work on ECAs as they emerge as standard practice to support. Weve partnered with top biopharma companies and are backed by leading venture capital firms to help increase our medical research and expand our product line.
In addition the US. The Aetion Evidence Platform analyzes data from the real world to produce transparent rapid and scientifically validated answers on safety effectiveness and value. This eBook will guide you through when where and how RWE studies have supported the.
1 in 2 of 2019 approved FDA submissions for new drugs and biologics included a real-world evidence study. Here at Aetion we are proud to have supported our clients in creating ECAs across multiple disease areas using the AEP. Founded by Harvard Medical School faculty members with decades of experience in epidemiology and health outcomes research Aetion informs health cares most critical decisionswhat works best for whom and.
The Aetion Evidence Platform analyzes data from the real world to produce transparent rapid and scientifically validated answers on treatments costs and outcomes. The technology is specifically built to engender trust and alignment across stakeholders bringing biopharma together. The Aetion Evidence Platform recognizes that every patient reacts to medications differently.
Its main product the Aetion Evidence Platform is able to analyze data from real-world evidence in order to help validate the safety effectiveness and value of certain treatments or procedures. With its patented rapid-cycle analytics technology the Aetion Evidence Platform utilizes the everyday clinical and financial interactions of the healthcare system to unlock essential evidence about the. Aetion is a health care technology company that delivers real-world evidence for life sciences payers providers and regulatory agencies.
Real World Evidence Solutions Rwe Analytics Aetion
 Enabling Essential Evidence For Healthcare Sebastian Schneeweiss Md
Enabling Essential Evidence For Healthcare Sebastian Schneeweiss Md
 New Enterprise Associates Backed Aetion Raises 110m To Advance Its Biotechnology Platform Cb Insights Research
New Enterprise Associates Backed Aetion Raises 110m To Advance Its Biotechnology Platform Cb Insights Research
 Real World Evidence Platform Rwe Solution Aetion
Real World Evidence Platform Rwe Solution Aetion
 Real World Evidence Solutions Rwe Analytics Aetion
Real World Evidence Solutions Rwe Analytics Aetion
Building A Rule Based Validation Framework Rvf For Real World Healthcare Data By Feng Zhang Aetion Technology Medium
 Real World Evidence Platform Aetion Adds 19m To Last Year S Series B Raise Mobihealthnews
Real World Evidence Platform Aetion Adds 19m To Last Year S Series B Raise Mobihealthnews
 Health Care Startup Aetion Raises 27 Million For Real World Evidence Platform Venturebeat
Health Care Startup Aetion Raises 27 Million For Real World Evidence Platform Venturebeat
 Aetion Announces 36 Million In Series B
Aetion Announces 36 Million In Series B
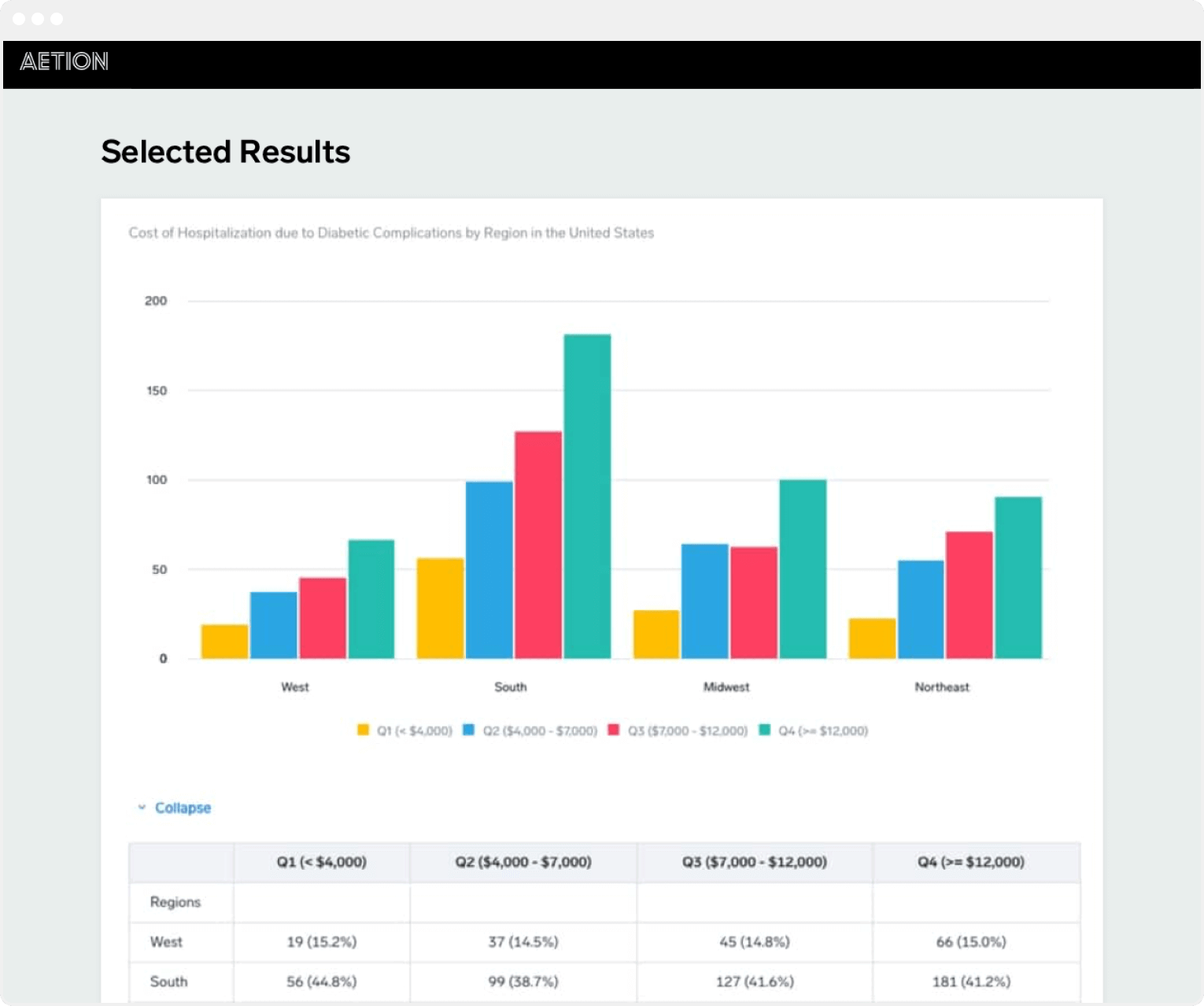 Real World Evidence Platform Rwe Solution Aetion
Real World Evidence Platform Rwe Solution Aetion
 Infographic What Role Does Rwe Play In Fda Approvals
Infographic What Role Does Rwe Play In Fda Approvals
 Real World Evidence Platform Developer Aetion Nabs Amgen Backed 36m Series B Fiercebiotech
Real World Evidence Platform Developer Aetion Nabs Amgen Backed 36m Series B Fiercebiotech
 Aetion Raises 36m For Evidence Analytics Platform For Value Based Care
Aetion Raises 36m For Evidence Analytics Platform For Value Based Care
 Real World Evidence Platform Rwe Solution Aetion
Real World Evidence Platform Rwe Solution Aetion

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.