APHINITY at 45 months median follow-up showed that pertuzumab added to adjuvant trastuzumab and chemotherapy significantly improved invasive diseasefree survival IDFS hazard ratio 081 95 CI 066 to 100 P 045 for patients with early human epidermal growth factor receptor 2 HER2positive breast cancer BC specifically those with node-positive or hormone receptor. The initial APHINITY study protocol protocol A included patients with either lymph node-positive or.
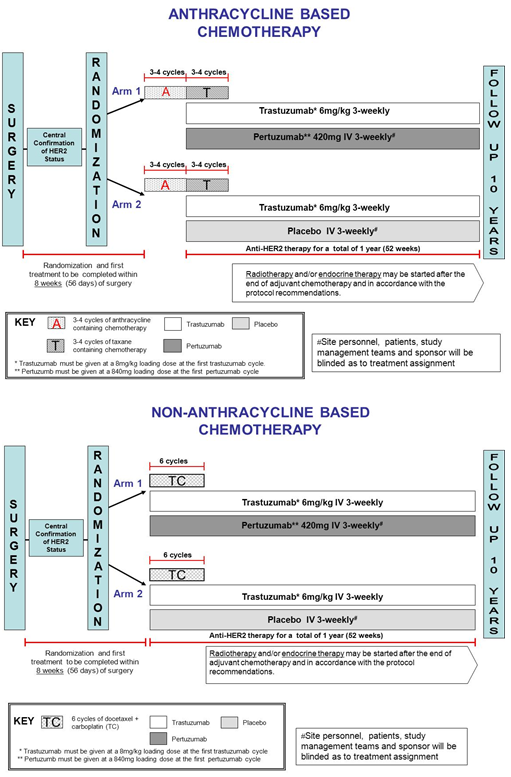
APHINITY Adjuvant Pertuzumab and Herceptin IN Initial TherapY in Breast Cancer NCT01358877 BO25126 BIG 4-11 is an international phase III randomised double-blind placebo-controlled two-arm study evaluating the efficacy and safety of Perjeta plus Herceptin and chemotherapy compared to Herceptin and chemotherapy as an adjuvant therapy in 4805 people with operable.

Aphinity trial breast cancer. What this trial did was capitalize on the results seen in the CLEOPATRA study. APHINITY at 45 months median follow-up showed that pertuzumab added to adjuvant trastuzumab and chemotherapy significantly improved invasive disease-free survival IDFS hazard ratio 081 95 CI 066 to 100 P 045 for patients with early human epidermal growth factor receptor 2 HER2-positive breast cancer BC specifically those with node-positive or hormone receptor HR. The primary efficacy endpoint of the APHINITY.
Adding pertuzumab to adjuvant therapy for high-risk HER2-positive early breast cancer in APHINITY. For those of us interested in breast cancer we have been waiting and waiting for the APHINITY trial data. Breast cancer were eligible for participation in the trial.
Expanding Treatment Options for HER2 Breast Cancer - Episode 3. A Study of Pertuzumab in Addition to Chemotherapy and Trastuzumab as Adjuvant Therapy in Participants With Human Epidermal Growth Receptor 2 HER2-Positive Primary Breast Cancer APHINITY The safety and scientific validity of this study is the responsibility of the study. Debu Tripathy MD.
Adding pertuzumab to standard trastuzumab-based adjuvant therapy significantly improved invasive disease-free survival IDFS in the APHINITY trial. The APHINITY trial was designed to test whether the addition of P to adjuvant TC improves pt outcomes. In previous trials P significantly prolonged progression free and overall survival and increased pCR rates when added to TC in pts with HER2-positive breast cancer BC.
The APHINITY trial was designed to test the addition of pertuzumab to a standard nonanthracycline or anthracycline-containing chemotherapy backbone along. HER2 positivity had to be centrally confirmed and was defined as an immunohistochemical score of 3 scores range from 0 to 3 with. 1 We dont have to wait anymore.
The APHINITY trial is an incredibly practice important study. APHINITY and ExteNET Trials for Early-Stage Breast Cancer. The APHINITY trial was initiated in November 2011 and included patients with HER2-positive early breast cancer who had previously had their.
SAN ANTONIO Data from six-year analysis of the APHINITY trial showed that adding pertuzumab to the previous standard of trastuzumab plus chemotherapy after surgery continued to reduce the risk of recurrence and death in patients with HER2-positive early breast cancer according to data presented at the 2019 San Antonio Breast Cancer Symposium held Dec. 33 The evidence for pertuzumab came from APHINITY an ongoing randomised controlled trial comparing pertuzumab plus trastuzumab and chemotherapy with placebo plus trastuzumab and chemotherapy in 4805 patients with HER2-positive early stage breast cancer who had had surgery. APHINITY Adjuvant Pertuzumab and Herceptin IN Initial TherapY in Breast Cancer NCT01358877 BO25126 BIG 4-11 is an international phase III randomised double-blind placebo-controlled two-arm study evaluating the efficacy and safety of Perjeta plus Herceptin and chemotherapy compared to Herceptin and chemotherapy as adjuvant therapy in 4805 people with operable HER2-positive eBC.
Data from six-year analysis of the APHINITY trial showed that adding pertuzumab to the previous standard of trastuzumab plus chemotherapy after surgery continued to reduce the risk of recurrence and death inpatients with HER2-positive early breast cancer according to data presented at the 2019 San Antonio Breast Cancer Symposium held Dec1014. SAN ANTONIO Texas An updated analysis of the APHINITY trial strengthens earlier findings showing the addition of pertuzumab Perjeta Roche. In this trial we investigated whether pertuzumab when added to adjuvant trastuzumab and chemotherapy improves outcomes among patients with HER2-positive early breast cancer.