Ongoing studies show that Keytruda and Tecentriq may not work well enough in some patients with cancer of the bladder and urinary tract. Ronald de Wit MD PhD.
 Esmo Merck S Keytruda Chemo Combo Shows Survival Trend But Can T Top Chemo In Bladder Cancer Fiercepharma
Esmo Merck S Keytruda Chemo Combo Shows Survival Trend But Can T Top Chemo In Bladder Cancer Fiercepharma
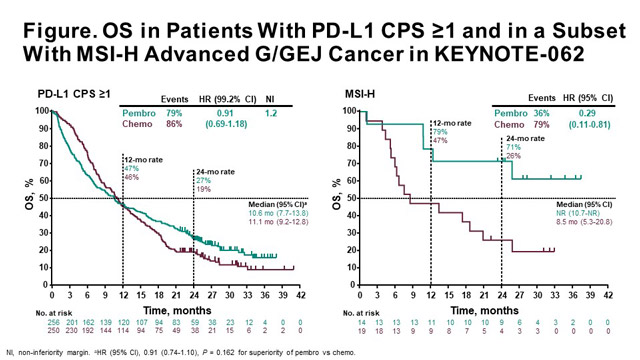
The 12-month survival was 444 with Keytruda and 298 with chemotherapy and the 24-month survival was 270 versus 143 with Keytruda and chemotherapy respectively.

Keytruda bladder cancer survival. Patients receiving Keytruda lived for 103 months median overall survival compared to 74 months with chemotherapy. Advertentie Unlimited access to Bladder Cancer market reports on 180 countries. After five years 232 percent of patients who had not received any treatment prior to Keytruda were alive.
Generally the EMA follows the CHMPs recommendation. Advertentie Bladder Cancer Patients from the world have benefited from Immucuras treatments. That compared with a 155 percent 5.
Request Free Consultation Today. If you have already had chemotherapy or have any other cancer your treatment with Keytruda or. Tap into millions of market reports with one search.
On Wednesday morning ODAC voted 5-3 in favor of keeping Keytrudas pembrolizumab accelerated approval alive as a first line bladder cancer treatment for those who are. Keytruda pembrolizumab produced better overall survival OS rates than chemotherapy for patients with recurrent advanced urothelial carcinoma. Patients treated with Keyturda had a median survival of 103 months compared to 73 months for those who received chemotherapy and PD-L1 expression status did not influence response to treatment with.
Bladder cancer survival statistics Cancer Research UK Accessed September 2020. Data show lower survival in some patients with low levels of cancer protein PD-L1. An equal number of patients 81 in either the Keytruda or chemotherapy groups had their cancer spread grow or get worse.
The EUROCARE-5 study on cancer survival in Europe 1999-2007. Database quality checks and. The 12-month survival was 444 with pembrolizumab and 298 with chemotherapy and the 24-month survival was 270 versus 143 with pembrolizumab and chemotherapy respectively.
Keytruda and Tecentriq will now only be used as a first treatment for this cancer in patients whose tests show a sufficient amount of a protein called PD-L1. Keytruda treatment resulted in a median OS of 103 months compared to 74 months in the chemotherapy arm. The phase III KEYNOTE-361 compared Keytruda plus chemotherapy cisplatin or carboplatin plus gemcitabine to standard-of-care chemotherapy and its two primary endpoints were overall survival OS.
Request Free Consultation Today. Advertentie Bladder Cancer Patients from the world have benefited from Immucuras treatments. The difference is Keytruda proved it could extend patients lives over.
IMT DCT is an advanced immunotherapy cancer treatment. Merck said data from an open-label Phase 3 trial of 542 advanced bladder cancer patients showed median survival of 103 months for Keytruda patients and 74 months for patients. IMT DCT is an advanced immunotherapy cancer treatment.
Tap into millions of market reports with one search. Show reduced survival with Keytruda pembrolizumab and Tecentriq atezolizumab when used as first-line treatments for urothelial cancer cancer of the bladder and. EMA restricts use of Keytruda and Tecentriq in bladder cancer.
Early data 1from two clinical trials. No statistically significant difference in progression-free survival PFS was observed between groups with those receiving Keytruda having a median PFS of 21 months compared to 33 months in the chemotherapy arm. AJCC Cancer Staging Manuel 8th Edition American Joint Committee on Cancer Springer 2017.
Although the KEYNOTE-361 study failed to meet its co-primary endpoints progression-free survival PFS and overall survival. Roches rival PD-L1 inhibitor Tecentriq suffered a similar fate when the FDA also limited its bladder cancer use in 2018. Advertentie Unlimited access to Bladder Cancer market reports on 180 countries.
Keytruda Bests Chemotherapy in Overall Survival Rates for Bladder Cancer.
 Keytruda Improves Survival Of Advanced Bladder Cancer Cancerconnect
Keytruda Improves Survival Of Advanced Bladder Cancer Cancerconnect
 Keytruda Approved For Bladder Cancer In Europe First To Prove Survival Benefit
Keytruda Approved For Bladder Cancer In Europe First To Prove Survival Benefit
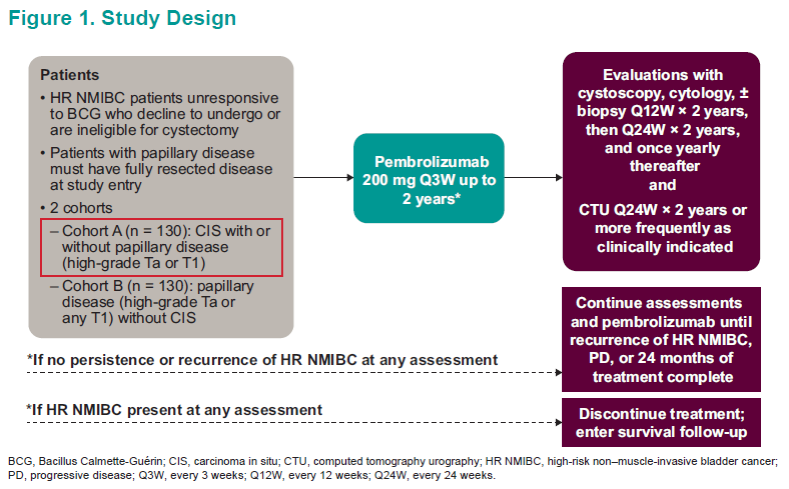 Asco 2019 Pembrolizumab For Patients With High Risk Non Muscle Invasive Bladder Cancer Unresponsive To Bacillus Calmette Guerin Updated Follow Up From Keynote 057
Asco 2019 Pembrolizumab For Patients With High Risk Non Muscle Invasive Bladder Cancer Unresponsive To Bacillus Calmette Guerin Updated Follow Up From Keynote 057
 Keytruda Fails To Hit Mark In First Line Bladder Cancer Treatment Trial Pharmalive
Keytruda Fails To Hit Mark In First Line Bladder Cancer Treatment Trial Pharmalive

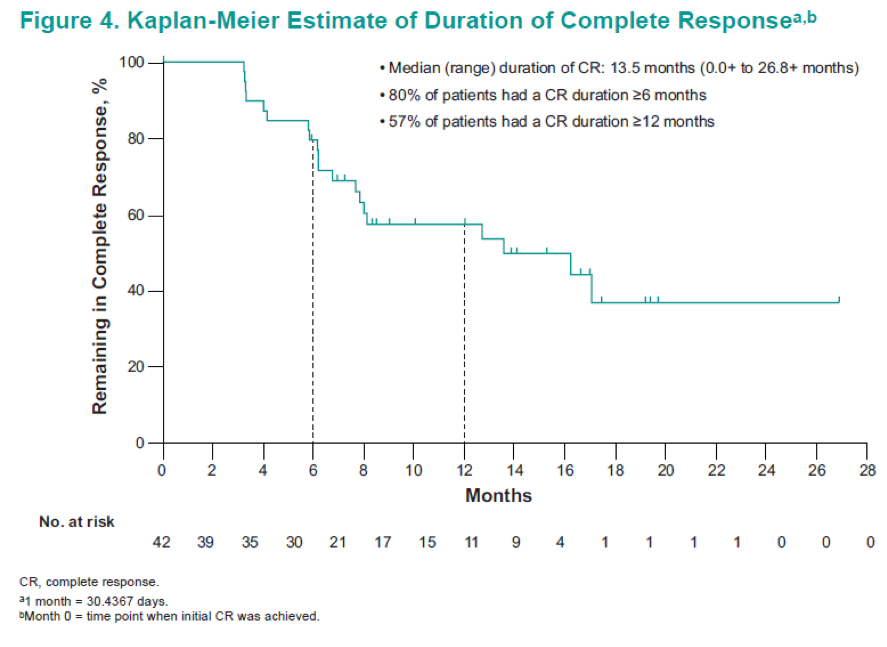 Asco 2019 Pembrolizumab For Patients With High Risk Non Muscle Invasive Bladder Cancer Unresponsive To Bacillus Calmette Guerin Updated Follow Up From Keynote 057
Asco 2019 Pembrolizumab For Patients With High Risk Non Muscle Invasive Bladder Cancer Unresponsive To Bacillus Calmette Guerin Updated Follow Up From Keynote 057
 Molecular Characterization Of Residual Bladder Cancer After Neoadjuvant Pembrolizumab European Urology
Molecular Characterization Of Residual Bladder Cancer After Neoadjuvant Pembrolizumab European Urology
 First Line Pembrolizumab Versus Chemotherapy In Patients
First Line Pembrolizumab Versus Chemotherapy In Patients
 Treatment Of Stage Iv Bladder Cancer Cancerconnect
Treatment Of Stage Iv Bladder Cancer Cancerconnect
 Pembrolizumab As Second Line Therapy For Advanced Urothelial Carcinoma Nejm
Pembrolizumab As Second Line Therapy For Advanced Urothelial Carcinoma Nejm

 Survival Bump In Bladder Cancer With Keytruda Medpage Today
Survival Bump In Bladder Cancer With Keytruda Medpage Today
 Updated Merck Ends Keytruda Trial Early With Data To Challenge Opdivo In Bladder Cancer Fiercepharma
Updated Merck Ends Keytruda Trial Early With Data To Challenge Opdivo In Bladder Cancer Fiercepharma
The Bladder Cancer Pendulum Swings Towards Bavencio Evaluate

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.